Background:
Patients with higher-risk MDS face limited treatment options and poor outcomes. While HMAs are approved as frontline therapy for higher-risk MDS, primary failure occurs in 50% of HMA-treated patients, and most responders progress within 2 years. There is an unmet need to improve the efficacy of AZA frontline therapy while maintaining a tolerable safety profile. Preclinical studies have demonstrated that the combination of selinexor and azacitidine synergistically inhibits MDS cell proliferation and arrests the cell cycle at the G2/M phase (Yixuan Guo, 2022). The combination of selinexor and azacitidine may be a promising approach for treating MDS. Here, we present this single-center, real-world evidence supporting the use of selinexor in combination with AZA in MDS patients.
Methods:
Medical records of 19 patients with MDS receiving selinexor in combination with AZA at the First Affiliated Hospital of Soochow University between Jan 2022 and March 2023 were reviewed. Inclusion criteria were a pathologically confirmed diagnosis of MDS, and receipt of ≥1 cycle of azacitidine in combination with selinexor. HMA-naïve patients and those previously treated with HMAs were included. Patients with a diagnosis other than MDS or progression to AML before treatment with selinexor + AZA were excluded. Patients received selinexor 40-60 mg QW orally and AZA 75 mg/m 2 intravenously or subcutaneously on days 1-5 or 7 of each 28-day cycle. Responses were evaluated per the International Working Group criteria.
Results:
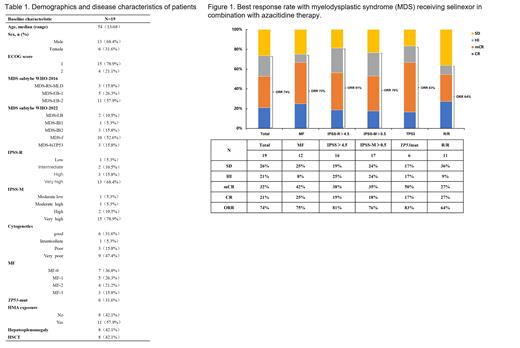
A total of 19 pts (median age, 54 y [range, 13-68 y]) were treated (Table 1). IPSS-R risk was high or very high in 15.8% and 68.4% of pts, respectively. IPSS-M risk was high or very high in 10.5% and 78.9% of pts, respectively. 57.9% of pts were previously treated with HMA. 63.2% had myelofibrosis, 42.1% had hepatosplenomegaly, 31.6% had a TP53 mutation, and 63.2% had poor or very poor-risk cytogenetics. As of Jun 10, 2023 at a median follow-up of 5.9 months (range 1.5-17.4 months), eight pts died (42.1%) due to disease progression. For the total cohort, the overall response rate (ORR) was 73%. In pts with worse survival prognosis, such as relapse and refractory, high and very high in IPSS-R, high and very high in IPSS-M, myelofibrosis and TP53 mut, the ORR was 64%, 81%, 76%, 75% and 83%, respectively (Figure 1). In seven pts with splenomegaly, six pts had reduced spleen volume, and one pt had no significant change by abdominal palpation or imaging examination. The 6-month overall survival (OS) was 74.7% in total pts, and 70.0% in pts previously exposed to HMA (Figure 2a, 2b). Selinexor + AZA therapy improved survival of patients with poor prognosis, such as TP53 mut (0.099), MF (+) (0.613), ≥high in IPSS-R (0.938) (Figure 2c, 2d, 2e). The survival of six pts who received transplantation after combination therapy was significantly prolonged, with 6-month OS was 83.3% (Figure 2f).
Conclusion:
Selinexor + AZA combination therapy was well tolerated with promising efficacy in patients with untreated or relapse/refractory MDS, including those with TP53 mutation, myelofibrosis, and higher risk in IPSS-R.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Selinexor (KPT-330) is a small molecule, oral, first-in-class, potent selective inhibitor of nuclear export (SINE) compound. Selinexor has received approval from the US Food and Drug Administration for RRMM patients and relapsed/refractory diffuse large B-cell lymphoma patients. A single-center, single-arm, phase 2 trial (ClinicalTrials.gov Identifier: NCT02228525) suggested that selinexor could be used in combination therapies in MDS and conclude that additional studies are needed.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal